Abstract
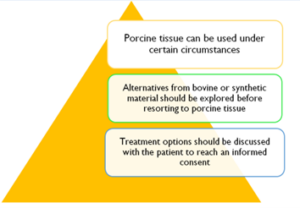
Valvular heart disease is a common condition. Mechanical and bioprosthetic valves are used to replace the valve when repair is not possible. In addition to the bovine source, bioprosthetic valves can also be made from porcine tissues.Therefore, replacement can pose challenges to the Muslim patient and the clinician aiming to reach an informed consent.Here we demonstrate theIslamic ruling on the use of porcine-derived heart valves and also we explore the available non-porcine options for Muslim patients. In Islam, the permissibility of porcine bioprosthetic heart valves involves two important concepts. The need for this particular treatment and the absence of an alternative option. In general, each patient’s scenario must be assessed individually, associated harms identified and weighed up by the patient and family in consult with the treating physician to reach an informed consent particularly if the use of porcine tissue is felt unavoidable.
Introduction
Patients religious beliefs have a strong influence on medical decisions and choice of treatment. This is of vital importance when the proposed treatment contains prohibited substances. Receiving such treatment can lead to religious distress and their provision could be perceived as an insensitive practice. The ingestion of porcine (pig-based) products is strictly prohibited in Islam. Many surgical prosthesis contain animal-derived material that commonly utilizes bovine or porcine products. The use of non-animal based prosthesis may be unavoidable in certain situations. Therefore, it is necessary for the treating physician to be aware of these issues as this will influence the consenting process.
It is well recognised that involving people in decisions about their health and care improves health and wellbeing. As a result, patients need information regarding benefits, risks and different treatment choices. Little is available in the literature to guide Muslim patients, suffering from valvular heart disease, to choose a treatment that is effective and at the same time fulfils their religious duty. The aim of this article is to provide the Islamic ruling on the use of porcine-derived heart valves and to explore the available non-porcine options for Muslim patients in order to assist physicians in delivering a patient-centred care taking into account patient-reported outcome measures and experience as part of the informed choice offered to patients.
The Islamic perspective
The prohibition on consumption of pork in Islamic law is well established. As the Qur’anic verses state:
Say, “I do not find within that which was revealed to me [anything] forbidden to one who would eat it unless it be a dead animal or blood spilled out or the flesh of swine – for indeed, it is impure – or it be [that slaughtered in] disobedience, dedicated to other than Allah. But whoever is forced [by necessity], neither desiring [it] nor transgressing [its limit], then indeed, your Lord is Forgiving and Merciful.” (6:145)
He has only forbidden to you dead animals, blood, the flesh of swine, and that which has been dedicated to other than Allah. But whoever is forced [by necessity], neither desiring [it] nor transgressing [its limit], there is no sin upon him. Indeed, Allah is Forgiving and Merciful. (2:173)
Although the use of porcine material in surgical prosthesis for the treatment of heart disease started in the twentieth century, Muslim scholars have established principles to draw on. In Al-Majmoo’, a well-established Islamic encyclopedia, Al-Nawawi (famous Islamic scholar from the thirteenth century) said
“If a person breaks a bone, it should be set using a pure bone. Our companions – i.e., the Shaafa’is – said: it is not permissible to set it using something impure, when one is able to use something pure instead. If he sets it using something impure the matter is subject to further discussion. If it needs to be set and he could not find anything pure to use instead, then he is excused. But if that was not necessary and there was something pure that could be used instead, then he has sinned and it must be removed if there is no fear that he may die or the limb may be damaged as a result.” End quote.
Based on this discussion, medical treatment by means of transplanting an animal organ of this type [i.e., from an impure animal e.g. pig] should meet two conditions:
1-The sick person should be in need of the transplant from the impure animal. This condition is met when specialist doctors testify that there is indeed such a need.
2-No pure organ is available that could be used instead.
If these two conditions are met, then there is nothing wrong with the surgeon transplanting this impure organ or part of it, and the presence of this impure organ in the patient’s body is not regarded as having any effect on his prayer or acts of worship for which purification is required, since there is a reason for which a concession is granted allowing it1.
The structure of the heart
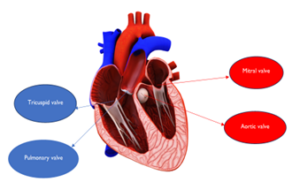
The heart (see Figure 1) has two parallel circuit systems one on the right and the other on the left. There are four heart valves that control blood flow. They open to allow flow in the correct direction and close to prevent blood leaking in the opposite direction. Each circuit has inlet and outlet valves. The inlet valves are the mitral valve on the left and tricuspid valve on the right whilst the outlet valves are the aortic valve on the left and pulmonary valve on the right. Disease affecting the ability of heart valves to open is termed stenosis and that resulting in valve incompetence is referred to as regurgitation.
Figure 1: The four heart valves
Valvular heart disease
Epidemiology
Valvular heart disease (VHD) is associated with reduced physical function, quality of life and contribute to increased mortality2. The incidence of VHD varies considerably around the world with rheumatic disease being the lead cause for valvular dysfunction in low-income countries and degenerative disease as the predominant pathology in high-income countries2.
Around 1.5m people aged above 65 in the UK are thought to have some form of VHD which includes aortic stenosis and mitral regurgitation. As a result of the aging population this number is likely to double in 2046 and reach 3.3m in the year 20563. If left untreated, VHD can lead to heart failure and is associated with an increased mortality risk4. Treatment be broadly divided into surgical repair or replacement and transcatheter treatment. The choice between surgical and transcatheter intervention is influenced by a number of factors including the specific valve in question, disease severity, anatomical consideration and the overall condition of the patient. When it comes to surgical replacement two types of valves are available with specific characteristics, advantages and disadvantages. Mechanical prosthesis have excellent long term durability but necessitate patients to be on long term anticoagulation (blood thinners) with a vitamin K antagonist (warfarin). This may be inconvenient and increases bleeding risk. In contrast, biological valves don’t require long term anticoagulation but at the same time are less durable. Biological valves are produced in most cases from bovine or porcine tissue. The decision to implant a bovine or a porcine based valve will take into account many factors accounting for anatomical and procedural characteristics. These factors should be discussed with patient and their family to help reach an informed consent.
Treatment options
Surgical treatment for VHD is well established with several decades of surgical experience. On the contrary transcatheter intervention is less established and despite producing favourable results in certain valvular pathologies the wide spread application across the spectrum of VHD is limited.
We aim in this article to give an overview of common valvular pathologies and give examples of biological valves from bovine tissue and synthetic material that are utilized in the treatment ofvalvular heart disease. The list is not intended to be exhaustive but will provide examples that may aid the treating physician and the patient to reach a decision which is patient-centred, avoids religious distress and possible litigation. Given the fact surgical treatment has developed over a long period of time resulting in a wide range of surgical prostheses available in the market we will provide a list of common biological surgical valves from bovine tissue. When it comes to transcatheter valves as the number of available prosthesis is limited we will discuss these options in more detail.
Aortic stenosis
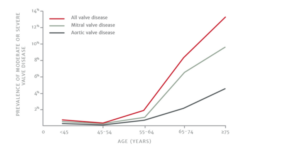
Aortic stenosis is a common condition where it is estimated that 1 in 8 people over the age of 75 develop aortic stenosis5(see figure 2). This pathology can also affect younger patients. The most common cause of aortic stenosis is valve calcification that occurs as part of the aging process. If left untreated half of the patients die or develop significant complications within 2 yrs6.
Figure 2. Prevalence of aortic stenosis
Treatment of aortic stenosis involves replacing the dysfunctional stenotic aortic valve. Depending on age, clinical and anatomical features the mode of replacement can be surgical (surgical aortic valve replacement SAVR) or transcatheter (Transcatheter aortic valve implant TAVI, also called TAVR) which is now a well-established minimally invasive treatment option. TAVI was first performed in Rouen by Cribier in 20027,8. Since then the number of TAVI implantations has increased exponentially around the world. Broadly speaking, TAVI prosthesis utilize a balloon expandable or a self-expanding design. The choice between a balloon expandable and a self-expanding valve depends on multiple clinical and anatomical variables. The heart team which normally includes of a surgeon, interventional cardiologist and an imaging cardiologist makes a recommendation but ultimately the treating physician will choose the most suitable prosthesis depending on clinical and anatomical features. Below is an example some of valve prostheses which are made using bovine pericardial tissue or synthetic material which should help the treating physician discuss with the patient the most appropriate valve choice.
Surgical prosthesis
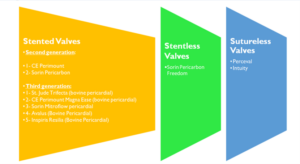
These can be divided broadly into stented, stentless and sutureless valves. The choice between these types will depend on clinical and anatomical features. Figures 3 will provide some examples of aforementioned stented, stentless and sutureless prosthesis from bovine or synthetic tissue
Figure 3. Examples of stented, stentless and sutureless prosthetic aortic valves from bovine or synthetic tissue.
Transcatheter prostheses
Edwards SAPIEN
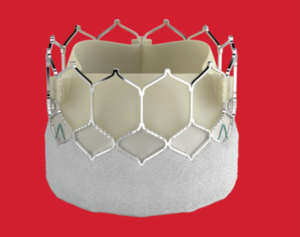
This is an example of a balloon expandable valve where the prosthesis is mounted on a balloon and is introduced to the body using a catheter commonly utilizing the common femoral artery (the vessel at the top of the leg). The latest iteration of the valve S3 ultra has a number of advantages to enhance safety clinical effectiveness. The EdawardsSapien valve (see Figure 4) has been studied in several clinical trials9-13, is widely used across the US and Europe, and has received a CE mark as well as FDA approval. The valve is available in four sizes (20mm, 23mm, 26mm, 29mm) and has an outer skirt to improve seal at the annulus (site of implantation and valve anchoring) and reduce the risk of paravalvular leak. It has a low profile access and a frame design with low frame height and open cell geometry allowing for better coronary access (access to the coronary arteries that supply blood to the surface of the heart). The use of the Edwards Sapien valve has been approved for use across the spectrum of high, intermediate and low risk from conventional surgical aortic replacement (SAVR).
Figure 4. Edward Sapien ultra TAVI prosthesis
ALLEGRA TAVI System TF
This is an example of a self-expanding valve. It has received CE mark for the treatment of severe calcified aortic valve stenosis in high-risk patients with elevated, surgical risk or in patients with a symptomatic degeneration of an aortic valve bioprosthesis. Allegra (see Figure 5) consists of a nitinol stent frame with a valve made of bovine pericardium. The delivery system uses a Permaflow technology, which ensures permanent blood flow condition throughout the process of valve positioning and deployment sequences. This principle allows early functionality of the valve. In contrast to other established TAVI systems the Allegra is a newly developed valve and therefore, the evidence for its use comes from smaller studies14-16. However, there are a number of functions that make the Allegra system a distinctive and a promising option. The concave shape of the stent frame with a tip deflection during diastole is designed to reduce shear stress and therefore, is likely to improve long term durability. The movable points at commissure reduce mechanical stress on the leaflet. In addition, the Allegra TAVI system has a frame with large cells and an outer skirt to reduce paravalvular leak.
Figure 5. Allegra TAVI system
Aortic regurgitation
Aortic regurgitation can be caused by disease of the aortic valve cusps and/or by disease of the aortic root and the ascending aorta. Degenerative disease is still the most common aetiology in high income countries accounting for two third of the cases17. Other causes included rheumatic and infective endocarditis. Treatment of aortic regurgitation is mainly surgical. Figure 3 lists examples of surgical protheses that can be utilized in the treatment of aortic regurgitation. The use of transcatheter heart valves to treat native pure aortic regurgitation has multiple challenges and is off label in certain cases that are deemed inoperable or at prohibitive risk from conventional surgery (SAVR).
Mitral stenosis
Mitral stenosis can be caused by a rheumatic, congenital or rarely by a degenerative process. Rheumatic fever is the leading cause of mitral stenosis across the world and remains a significant problem in the developing world affecting young patients, whereas its prevalence in industrialized countries has significantly declined18-20.
Treatment involves mitral valve surgery and percutaneous/transcatheter that can be utilized in certain situations. Figure 6 lists common mitral prosthesis from bovine tissue. The percutaneous option involves a balloon which comes from synthetic material to dilate the stenotic (diseased) valve. In selected cases some TAVI prostheses were used in the mitral position to treat patients who were deemed high risk for conventional surgery and unsuitable for balloon intervention.
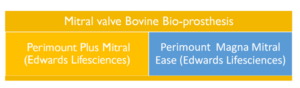
Figure 6. Mitral valve prosthesis from bovine tissue commercially available in current practice
Mitral regurgitation
This is the second most common valve pathology in Europe21,22. Treatment of mitral regurgitation is surgical with valve repair or replacement. Surgical prostheses have been described in figure 6.
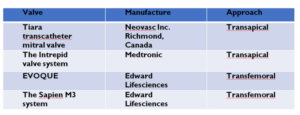
However, if the operative risk is high, transcatheter options can be considered in certain cases where transcatheter edge to edge repair (TEER) is the most evidenced. This option involves synthetic material rather than bovine or porcine tissue. Example of these devices are listed below. To our knowledge, no designated mitral valve prosthesis from bovine material is currently available in the market. However, several devices (see table 1) are currently under clinical evaluation and utilizes the transapical and transfemoral routes23. The transapical approach involves introducing the valve using a small incision from the apex of the heart. The transfemoral route makes use of the femoral artery.
Mitral clip
The device is an example of the edge to edge repair and consists of a small metal clip covered with a polyester fabric that is implanted on your mitral valve using a designated catheter system.
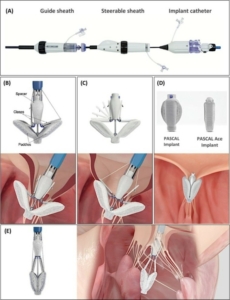
Pascal system
This is another example of the TEER system that similarly to the Mitra clip device uses synthetic material. It comes in two sizes pascal and pascal Ace (Figure 7).
Figure 7. Pascal and pascal Ace systems
Table 1: Examples of transcatheter mitral prosthesis from bovine tissues under clinical evaluation
Tricuspid stenosis
This pathology is often combined with tricuspid regurgitation and is usually associated with disease of the mitral or aortic valve. Treatment is predominantly surgical. Figure 8 describes common surgical prosthesis.
Figure 8. Examples of tricuspid valve prosthesis from bovine tissue commercially available in current practice.
Tricuspid regurgitation
The incidence of significant tricuspid regurgitation increases with age affecting 4% of the population aged 75 or over23. The most common aetiology of tricuspid regurgitation is secondary to disease affecting the right side of the heart (right ventricle/atrium) and is often associated with valvular heart disease of the mitral and or the aortic valve. If left untreated severe tricuspid regurgitation is associated with reduced survival23-26 and increased incidence of heart failure28,29. Surgical treatment is recommended in all symptomatic patients. See figure 8 for a list of devices.
Transcatheter tricuspid valve intervention (TTVI) is under evaluation and continuous development. It involves placing a ring around the valve, bringing together the valve leaflets or using a designated prosthetic valve. Below are few examples of each type
Cardioband
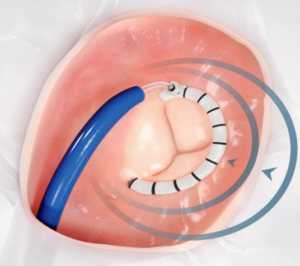
This design utilizes a ring manufactured using synthetic material and is placed using a transcatheter system. (See Figure 9).
Figure 9. Example of cardioband system
Triclip
This device is an example of the TEER system that consists of a metal clip and synthetic material
Pascal system
Another device that utilizes the TEER approach and made of a metal clip and synthetic material. As in the mitral system it comes in two sizes pascal and pascal ACE. (See Figure 7).
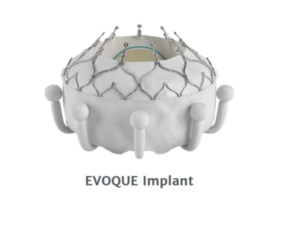
EVOQUE tricuspid valve replacement system
This is an investigational device that has a prosthetic valve made using bovine tissue and implanted into the patient tricuspid valve using a transcatheter approach (see Figure 10).
Summary
Valves are the main component of the heart structure. They work continuously and efficiently in a synchronised harmonic way to maintain a coordinated flow of blood in the right direction. Valvular heart disease is common affecting 1 in 40 below the age of 40 rising to 1 in 10 in people aged 75 or more and can range from being asymptomatic to severe and life-threatening5. Pharmacological treatment options were initially developed to alleviate symptoms, however, given the mechanical nature of the disease, over time there has been an increasing focus on the surgical repair or replacement as a curative option. Transcatheter treatment has gained increasing momentum particularly in patients at a high risk of surgical intervention and in certain cases where clinical and anatomical factors are favourable. Bioprosthetic tissue derived from bovine or porcine material has been used in surgical and transcatheter heart valves.
The prohibition of consumption of pork in the Islamic law is well established. The permissibility of porcine bioprosthetic heart valves involves two important concepts. The need for this particular treatment and the absence of an alternative option1. In general, each individual patient’s scenario must be assessed, associated harms identified and weighed up by the patient and family in consult with the treating physician to reach an informed consent particularly if the use of porcine tissue is felt unavoidable. With the advancing in technology and development of new valve prosthesis alternatives to porcine tissue are becoming more readily available and these should be explored when treating Muslim patients.
Key messages
Funding disclosures or relationships with industry:
Dr.Hazim Rahbi received an educational grant from Edwards life science. Dr. Firas Aljanadi: No disclosures or Conflict of interest.
References
- Islam Q&A. https://islamqa.info/en/answers/93212/is-it-permissible-to-do-an-operation-on-the-heart-valves-using-pigski
- Sean Coffey, Ross Roberts-Thomson, Alex Brown, Jonathan Carapetis, Mao Chen, Maurice Enriquez-Sarano, Liesl Zühlke, Bernard D. Prendergas Nature Reviews Cardiology volume 18, pages853–864 (2021)
- D’Arcy L, Coffey S, Loudon M et al (2016) Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the Ox VALVE Population Cohort Study. European Heart Journal.
- Nishimura RA, Otto CM, Bonow RO, et al (2014) AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol; 63:2438–88
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11.
- Otto C. Timing of aortic valve surgery. Heart 2000;84(2):211-218
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:15971607.
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:21872198.
- Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG, PARTNER 1 trial Investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:24772484
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:16091620.
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR, PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in lowrisk patients. N Engl J Med 2019;380:16951705.
- Wenaweser P, Stortecky S, Schütz T, Praz F, Gloekler S, Windecker S, Elsässer A. Transcatheter aortic valve implantation with the NVT Allegra transcatheter heart valve system: first-in-human experience with a novel self-expanding transcatheter heart valve. 2016 May 17;12(1):71-7. doi: 10.4244/EIJV12I1A13.
- Dariusz Jagielak, Aleksandra Stanska , Andrzej Klapkowski , Maciej Brzezinski , Maciej Kowalik , Dariusz Ciecwierz , Milosz Jaguszewski , Marcin Fijalkowski. Transfermoral aortic valve implantation using self-expanding New Valve Technology (NVT) Allegra bioprosthesis: A pilot prospective study. Cardiol J. 2021;28(3):384-390.
- Ulrich Schäfer, Christian Butter, Martin Landt, Christian Frerker, Hendrik Treede, Johannes Schirmer, Cornel Koban, AbdelhakimAllali, Tobias Schmidt, EfstratiosCharitos, Olga Nikolayevska, Lenard Conradi. One-year clinical outcomes of a novel transcatheter heart valve to treat degenerated surgical valves: the VIVALL study. EuroIntervention2022;17:1077-1080.
- Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, De Bonis M, Tribouilloy C, Evangelista A, Bogachev-Prokophiev A, Apor A, Ince H, Laroche C, Popescu BA, Pierard L, Haude M, Hindricks G, Ruschitzka F, Windecker S, Bax JJ, Maggioni A, Vahanian A, EORP VHD II Investigators. Contemporary presentation and management of valvular heart disease: The EUR Observational Research Programme Valvular Heart Disease II Survey. Circulation 2019;140:1156-1169.
- Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, Alahdab F, Alashi A, Alipour V, Arabloo J, Azari S, Barthelemy CM, Benziger CP, Berman AE, Bijani A, Carrero JJ, Carvalho F, Daryani A, Duraes AR, Esteghamati A, Farid TA, Farzadfar F, Fernandes E, Filip I, Gad MM, Hamidi S, Hay SI, Ilesanmi OS, NaghibiIrvani SS, Jurisson M, Kasaeian A, Kengne AP, Khan AR, Kisa A, Kisa S, Kolte D, Manafi N, Manafi A, Mensah GA, Mirrakhimov EM, Mohammad Y, Mokdad AH, Negoi RI, Thi Nguyen HL, Nguyen TH, Nixon MR, Otto CM, Patel S, Pilgrim T, Radfar A, Rawaf DL, Rawaf S, Rawasia WF, Rezapour A, Roever L, Saad AM, Saadatagah S, Senthilkumaran S, Sliwa K, Tesfay BE, Tran BX, Ullah I, Vaduganathan M, Vasankari TJ, Wolfe CDA, Yonemoto N, Roth GA, Global Burden of Disease Study Nonrheumatic Valve Disease Collaborators. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation 2020;141:16701680.
- Kingue S, Ba SA, Balde D, Diarra MB, Anzouan-Kacou JB, Anisubia B, Damorou JM, Ndobo P, Menanga A, Kane A, Kakou-Guikahue M, Kenfack M, Metogo B, Chelo D, Yangnigni E, Tantchou C, Bertrand E, Monsuez JJ, Working Group on Tropical Cardiology of the Socie´te´ franc¸aise de cardiologie. The VALVAFRIC study: a registry of rheumatic heart disease in Western and Central Africa. Arch Cardiovasc Dis 2016;109:321329.
- Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol2014;30:962970.
- Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, De Bonis M, Tribouilloy C, Evangelista A, Bogachev-Prokophiev A, Apor A, Ince H, Laroche C, Popescu BA, Pierard L, Haude M, Hindricks G, Ruschitzka F, Windecker S, Bax JJ, Maggioni A, Vahanian A, EORP VHD II Investigators. Contemporary presentation and management of valvular heart disease: The EUR Observational Research Programme Valvular Heart Disease II Survey. Circulation 2019;140:11561169.
- Cahill TJ, Prothero A, Wilson J, Kennedy A, Brubert J, Masters M, Newton JD, Dawkins S, Enriquez-Sarano M, Prendergast BD, Myerson SG. Community prevalence, mechanisms and outcome of mitral or tricuspid regurgitation. Heart 2021 doi: 10.1136/heartjnl-2020-318482
- Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, Mahoney DW, Enriquez-Sarano M. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging 2019;12:433-442.
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on longterm survival. J Am CollCardiol2004;43:405409.
- Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, Pislaru S, Park S, Mahoney DW, Biner S, Enriquez-Sarano M. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:1185-1194.
- Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv-Baran T, Richert E, Keren G, Banai S. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging 2020;21:157-165
- Benfari G, Antoine C, Miller WL, Thapa P, Topilsky Y, Rossi A, Michelena HI, Pislaru S, Enriquez-Sarano M. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation 2019;140:196-206.
- Topilsky Y, Inojosa JM, Benfari G, Vaturi O, Maltais S, Michelena H, Mankad S, Enriquez-Sarano M. Clinical presentation and outcome of tricuspid regurgitation in patients with systolic dysfunction. Eur Heart J 2018;39:3584-3592.